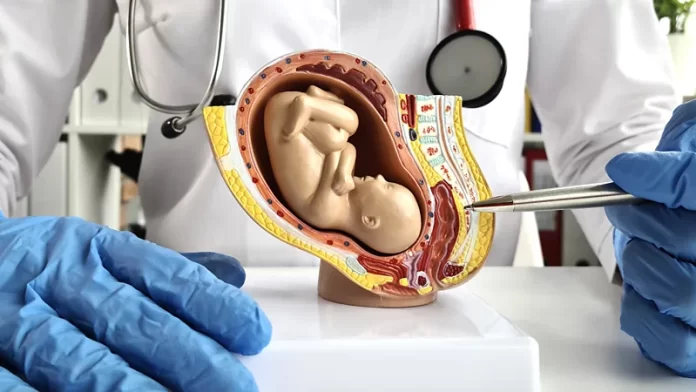
It sounds like a plot straight out of a bad science fiction movie – human babies taken from their mothers’ wombs and grown inside fluid-filled pods instead. Yet that is exactly what scientists at the Children’s Hospital of Philadelphia (CHOP) in Pennsylvania in the US propose doing for infants at risk of extreme prematurity.
They are developing what they refer to as an “artificial womb”, or extra-uterine environment for newborn development (Extend) to be precise. Extend is not intended to grow a foetus from conception to birth – that would be impossible even if it was desirable. Instead, it is intended to help boost the survival rate of extremely premature infants, who face a plethora of possible health effects throughout their lives.
A typical healthy pregnancy lasts around 40 weeks, with babies considered full term at 37 weeks. However sometimes complications occur in pregnancy that may result in a baby having to be delivered early.
Luckily, thanks to huge advances in neonatal medicine over the last few decades, most premature infants survive and are discharged with few complications. The most recent data shows that even 30% of 22-week-gestation patients survive if given intensive care.
“Honestly, 28 weekers and even 27 weekers do overall very well,” says Stephanie Kukora, a neonatologist at Children’s Mercy Hospital in Kansas City.
“It’s really the babies born at 22 to 23 weeks that the outcomes are so severe that we are not sure whether the quality of life that they attain is acceptable.”
Babies born on the cusp of viability often face severe health challenges. These infants weigh less than 2lb (900g) at birth, and critical organs such as the heart, the lungs, the digestive organs, and the brain are not yet developed enough to keep the baby alive without intensive medical care.
Short-term complications which frequently arise include necrotising enterocolitis (NEC), a serious illness in which tissues in the intestine (gut) become inflamed and start to die. Infants of this age are also very prone to infection, sepsis and septic shock – a life-threatening drop in blood pressure that can damage the lungs, kidneys, liver and other organs.
Meanwhile long-term issues that can affect extremely premature babies include cerebral palsy, moderate to severe learning difficulties, vision and hearing problems, and asthma.
The idea behind artificial wombs and placentas is to take the lungs out of the equation all together
Even the very technology designed to save the babies’ life – oxygen support and ventilation – can harm the infants’ fragile lungs.
“At that early gestational age the lungs are still developing and should be filled with fluid,” says George Mychaliska, a professor of surgery and obstetrics and gynaecology at Michigan University’s C S Mott Children’s Hospital.
“But when they’re born very prematurely, we put an endotracheal tube in their trachea, and we force air and oxygen at high tension and pressure into their lungs – that is well documented to cause injury.”
Over time the injuries lead to scarring of the lungs and a condition known as bronchopulmonary dysplasia, or chronic lung disease. Children often leave hospital needing long-term oxygen support and require mechanical ventilation for the rest of their lives. Ventilation can also raise the risk of retinal blindness. The blood vessels that feed the eye’s retina aren’t fully formed until close to birth. Too much oxygen can trigger the growth of new, abnormal blood vessels, which can ultimately lead to retinal detachment.
The idea behind artificial wombs and placentas is to take the lungs out of the equation all together, allowing time for the foetus to continue developing in a safe environment until the baby is ready to take its first breath.
There are three main groups working on the technology. All three take their inspiration from an existing therapy called extracorporeal membrane oxygenation (Ecmo), a type of artificial life support that can help a person whose lungs and heart aren’t functioning properly. In Ecmo, blood is pumped outside of the patient’s body to a machine that removes carbon dioxide and adds oxygen. The oxygenated blood is then sent back to tissues in the body.
This method allows the blood to “bypass” the heart and lungs, allowing these organs to rest and heal. Although Ecmo can be used on older babies, it isn’t suitable for extremely premature infants. All three teams are trying to miniaturise and adapt the technology.
However, there are subtle differences between the different devices in development.
Scientists at CHOP, led by foetal surgeon Alan Flake, plan to submerge premature babies in fluid-filled pods designed to mimic the amniotic fluid of the womb. Surgeons would then connect the tiny blood vessels of the baby’s umbilical cord to an Ecmo-like device. The blood is pumped around the system using the foetal heart, just like in nature.
In 2017, Flake and his colleagues took eight premature lambs of an equivalent gestational age to 23-to-24-week-old human foetuses and kept them alive for four weeks using the artificial womb. During this time the lambs seemed to develop normally, even growing wool.
George Mychaliska’s team at the University of Michigan, on the other hand, are developing what they call an artificial placenta. Rather than submerging the whole foetus in fluid, they plan on using breathing tubes to fill the lungs of the infant with a specially developed fluid. Their system drains blood from the heart via the jugular vein, similar to traditional Ecmo machines, but returns oxygenated blood via the umbilical vein.
Premature lambs maintained on the machine survived for 16 days before being safely transferred to mechanical ventilation
“I wanted a platform that’s readily available to most babies, and that could be used in existing neonatal intensive-care units,” says Mychaliska.
“The technology isn’t intended to replace the myriad functions of the placenta. It’s focusing on gas exchange and maintaining blood pressure, heart rate and foetal circulation while the premature organs are protected and continue developing.”
In a recent trial of the artificial placenta, premature lambs maintained on the machine survived for 16 days before being safely transferred to mechanical ventilation. During this time their lungs, brains and other organs continued to develop well.
The third group, a team from Australia and Japan, is developing an artificial womb called ex vivo uterine environment (Eve) therapy. It is aimed at treating more premature and sick foetuses than the other two groups.
“We’re now at a point where we can take a 500g [lamb] foetus and maintain it in what I would describe as a broadly normal physiological state for two weeks at a time,” says Matt Kemp, professor of obstetrics and gynaecology at the National University of Singapore, who leads Eve.
“That’s a pretty neat achievement, but on the flip side, the growth of these foetuses is abnormal.”
Most of the trials conducted using artificial placentas/wombs are on lamb foetuses that are otherwise healthy and would have gone through to term if left undisturbed. The problem is that extremely premature babies are often born early because of health complications arising either in the mother or the foetus itself. They are therefore more difficult to treat.
“In the one experiment that we’ve done with quite compromised foetuses, those animals are much more difficult to manage,” says Kemp.
We think that it’s pretty clear that a very small foetus doesn’t have the ability to direct its own growth in a normal fashion
“Their growth is far worse, and their blood pressures and flow is much, much more difficult to keep normal. So, it’s a case of – yes, we’re making some good progress, but we’ve got a bunch of stuff we need to figure out.”
So how soon will we see artificial placentas and wombs in hospitals? CHOP is probably furthest along the development pipeline. The team recently applied to the Federal Drug Administration (FDA) for permission to begin human trials of Extend. Mychaliska, on the other hand, hopes to move to human clinical trials in around three or four years, after his team have miniaturised their system further to cope with the tiny blood vessels of a human neonate.
However, Kemp still thinks there are fundamental gaps in our knowledge of how foetuses grow in artificial wombs that need filling in before we move to trials.
“We think that it’s pretty clear that a very small foetus doesn’t have the ability to direct its own growth in a normal fashion, and that’s exacerbated when it is sick,” says Kemp.
“So we’re trying to unpack the involvement of the placenta in driving those normal growth processes. This is sort of where we’re up to. That’s a pretty big task, to put it mildly.”
There are ethical considerations too. In a recent article, Stephanie Kukora argues that there are subtle differences between the distinct technologies that create unique ethical challenges. For instance, as both the EVE and CHOP teams’ artificial wombs require fitting a cannula to the umbilical cord, babies need to be immediately transferred from the mother to the device, as the umbilical artery closes quickly after birth. Mothers who otherwise could have delivered vaginally would therefore need to have an early Caesarean section.
“When you have a Caesarean section that early, they can’t do it the way that they do it at term,” says Kukora.
“It involves an incision that goes through the muscular layer of the uterus, and that can have an impact on future pregnancies, such as whether they can go to term and whether they can be delivered vaginally.”
There are more risks associated with this procedure compared to a vaginal birth, which raises issues to do with informed consent.
“I think that one of the biggest ones is how we will approach expectant parents about doing this trial,” says Kukora.
“You can imagine a parent who’s facing this really sad situation, who’s just been counselled about the poor outcomes at 22 weeks, and who might be really excited for something new even if it’s untested. Parents will do anything for their infant.”
Babies who would otherwise have done well on traditional therapies could be treated on a new untested technology, whose risks are much less quantified
Another issue with immediately transferring a baby onto the Extend system is that there is no opportunity to assess how that baby would have done on conventional therapy.
“You don’t have a lot of data apart from the gestational age to decide who goes on the Extend system – because the baby’s not born yet, so you don’t know how they’re doing,” says Mychaliska.
This may mean that babies who would otherwise have done well on traditional therapies could be treated on a new untested technology, whose risks are much less quantified. However, Mychaliska believes Extend would be beneficial for the most premature infants at 22-23 weeks gestational age, who are known to suffer high mortality and morbidity.
As it drains blood from the jugular vein rather than the umbilical artery, doctors have more time to place babies on Mychaliska’s artificial placenta. This allows physicians to “risk stratify” babies after birth, with the aim that only the sickest infants are transferred to the treatment arm of the trial. Infants could also potentially be treated using conventional therapy first, before being transferred to the artificial placenta at a later date if they weren’t doing well. Unlike the other two technologies, mothers can also deliver their babies vaginally.
Whichever technology reaches trials first, the first participants in the trials are likely to be babies born before 24 weeks that have a very poor chance of survival with a good outcome using conventional treatment.
“I think the technology will revolutionise the field of prematurity, and the artificial placenta and Extend approaches will be complimentary in clinical practice,” says Mychaliska.
“But it’s also not without potential risks which need to be assessed in an initial trial of safety. I think the initial application of this technology should be on babies that have a poor chance of survival, and then expanded to more premature infants once we determine the risks and efficacy of this technology.”
If successful, all three technologies will offer a much-needed lifeline of hope to parents who unexpectedly go into premature labour.